CEFALY Treatment and prevention of migraines

CEFALY Electrodes
What is CEFALY?

Who is CEFALY for?

CEFALY electrodes

How does CEFALY work?
How to use CEFALY?

Cleansing the skin
Carefully cleanse the skin on the forehead using a “Prepare” cleansing wipe or soap and water. This step is essential for degreasing the skin and ensuring that the electrode will effectively stick to the skin.

Positioning the electrode
Use a mirror to help you place the electrode on your forehead correctly. The narrowest part of the electrode should point down towards your nose. The lower, curved edge should be placed just above the top of the eyebrows.
Get Electrodes
Placing CEFALY
Hold the device and bring the rear side of the device with the two magnets against the central part of the electrode. The device is attracted by the two contact zones of the electrode and gets automatically into position.
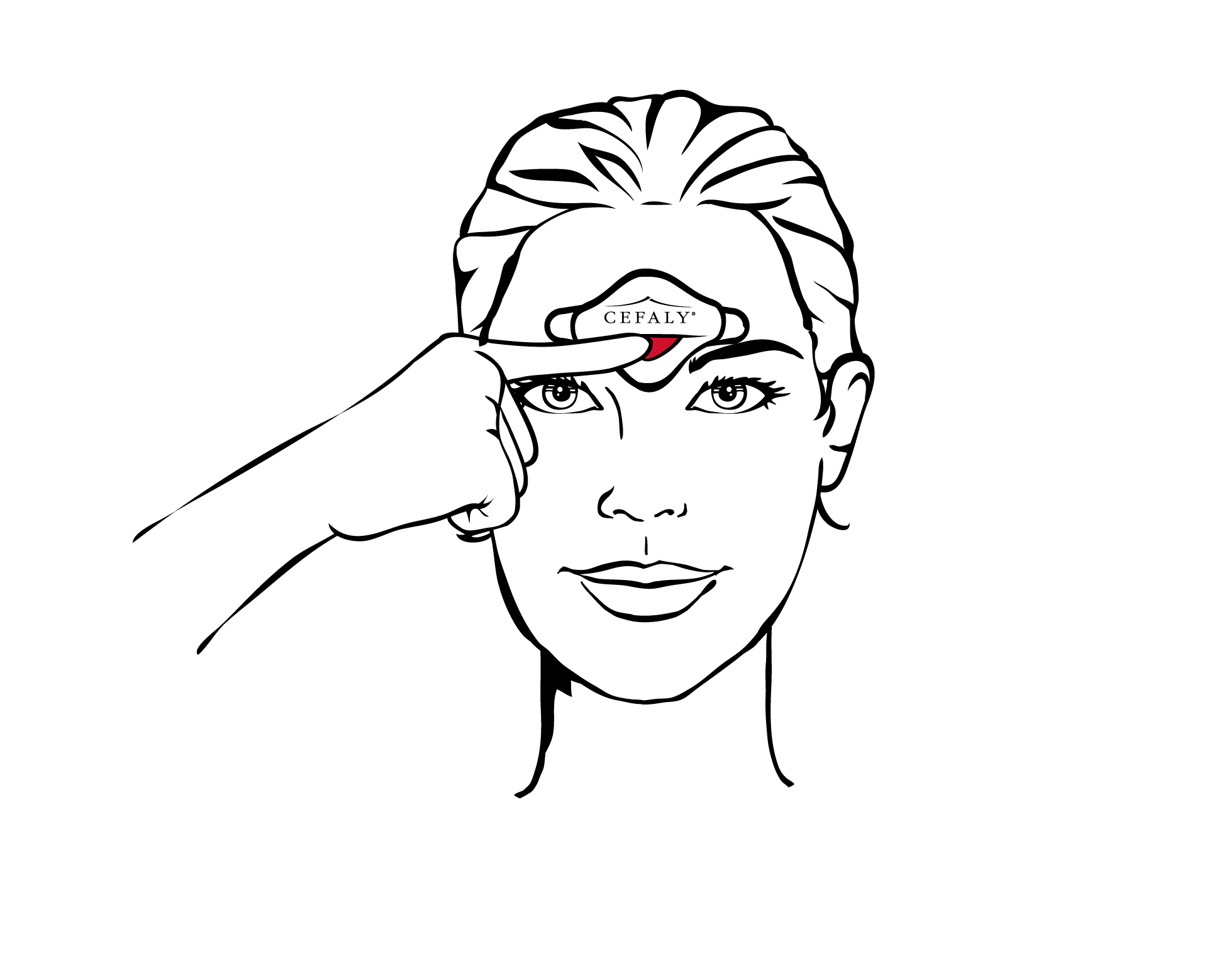
Starting
Press the button to start the session. A single press starts programme 1 (crisis treatment): you will hear one beep. Two presses, one after the other, starts programme 2 (prevention): you will hear two beeps. Three presses, one after the other, starts programme 3 (antistress): you will hear three beeps.
Adjusting the intensity
The intensity and the tingling will increase. If it becomes a little too much, press the button again to stabilize the intensity. It will not increase again over the 20 minutes of treatment. But you should make progress wherever possible! As you repeat your CEFALY sessions, your tolerance of the intensity will increase. You will constantly increase the intensity and the sessions will become more and more effective.

Duration and ending the session
During the twenty-minute CEFALY session, relax or carry out your normal activities. The session ends after twenty minutes and the device automatically switches off. Remove the device by pulling it forwards.
Get CEFALY Enhanced TodayEfficacy for CEFALY Users

Indications
What is it?
Attacks come on without any prior warning and can vary in length (4 to 72 hours). Between attacks, there are no symptoms. The pain is violent, pulsating, usually on one side of the head and made worse by physical activity, often being accompanied by nausea or vomiting. Sufferers find noise and light difficult to tolerate.
CEFALY?
Is indicated when attacks are frequent (several times a month). CEFALY's effectiveness will first and foremost be preventative in order to significantly reduce the frequency of migraine attacks. It can also reduce pain during an attack.
Benefit?
Significant improvement for 2 patients out of 3.
What is it?
The attack is preceded by an aura (neurological symptoms) such as tingling in one part of the body or a blind spot (scotoma) in the field of vision. The actual attack can then vary in length (4 to 72 hours). Between attacks, there are no symptoms. The pain is violent, pulsating, usually on one side of the head and made worse by physical activity, often being accompanied by nausea or vomiting. Sufferers find noise and light difficult to tolerate.
Cefaly?
Is indicated when attacks are frequent (several times a month). Cefaly’s effectiveness will first and foremost be preventative in order to significantly reduce the frequency of migraine attacks. It can also reduce pain during an attack.
Benefit?
Significant improvement for 2 patients out of 3
I want to try CEFALY
What is it?
The migraine attack is preceded by a visual aura which often appears in the form of a blind spot (scotoma) in the field of vision, sometimes with flashing outlines. Some patients also speak of an ophthalmic migraine when the pulsating pain of the attack is located around one eye.
Cefaly?
Is indicated when attacks are frequent (several times a month). Cefaly's effectiveness will first and foremost be preventative in order to significantly reduce the frequency of migraine attacks. It can also reduce pain during an attack.
Benefit?
Significant improvement for 2 patients out of 3.
I want to try CEFALY
What is it?
These are migraines (common or with aura) that are characterised by attacks of varying length (4 to 72 hours). Between attacks, there are no symptoms. The frequency of the attacks varies, ranging from one per month (or less) to several times a week.
Cefaly?
Is indicated when attacks are frequent (several times a month). Cefaly's effectiveness will first and foremost be preventative in order to significantly reduce the frequency of migraine attacks. It can also reduce pain during an attack.
Benefit?
Significant improvement for 2 patients out of 3.
What is it?
This is a migraine that has been ongoing for some time and the attacks from it have become very frequent. The headaches are present at least half of the time or more (the patient suffers headaches on more than 14 days in a month).
Cefaly?
Has proven its effectiveness in episodic migraines. For chronic migraines, we are not yet entirely sure - however many patients are satisfied with the effectiveness of Cefaly. Especially with the help it brings in reducing the consumption of medications.
Benefit?
Probable improvement for more than 50% of all cases.
What is it?
Menstrual migraines occurs only around the time of a woman's monthly period. The hormonal factor is the crucial one here. The signs of migraine are classical with the feature of being regulated by the menstrual cycle.
Cefaly?
Cefaly is useful for relief, especially when it is used several days before the start of the painful period.
Benefit?
Improvement for more than 50% of all cases.
What is it?
Tight or pressure-type pain, often described like a bar located on the left and right of the anterior part of the skull. Anterior tension type headaches do not get worse with physical activity, nor are they accompanied by vomiting or visual disturbances.
Cefaly?
The key indication for Cefaly is migraine, however data indicates that it can be extremely useful for some patients who have frontal tension headaches.
Benefit?
Significant improvement for more than 50% of all cases.
What is it?
Tight or pressure-type pain, often described like a heaviness located at the back and the base of the skull. Posterior tension type headaches do not get worse with physical exertion, nor are they accompanied by vomiting or visual disturbances.
Cefaly?
For this condition, Cefaly must be used with the occipital electrode
Benefit?
Significant improvement for more than 50% of all cases.
What is it?
These are almost daily headaches that have been occurring for a long time. The pain is felt like a weight, tightness or pressure, like a bar or a vice crushing the head. This type of headache is often accompanied by the excessive consumption of medications, which can increase the number of headaches (vicious circle).
Cefaly?
Cefaly is particularly useful in this instance. It reduces the pain and the consumption of medications. Its regular use allows sufferers to break out of the vicious circle caused by using too many medications.
Benefit?
Significant improvement for more than 50% of all cases.
What is it?
This is an irritation or inflammation of the occipital nerve. The sharp pain is like a burn or electric shock, travelling from the top of the neck to the crown of the head. It starts at the cervical spine and is generally caused by arthrosis, lesions secondary to an accident (whiplash), etc. This type of neuralgia is not very common and is often confused with migraine when the pain starts in the occipital region.
Cefaly?
Cefaly has an occipital electrode (Arnold kit) that is designed to specifically work at the level of the major occipital nerve (or Arnold’s nerve).
Benefit?
Significant improvement for more than 50% of all cases.
Best Safety
More than 200,000 Cefaly devices in use. Data since 2008.

The side effects of Cefaly
- Observed in 4.3% of patients
- Mild and completely reversible
- Most common: intolerance to the feeling of CEFALY on the forehead
- Most severe: allergic skin reaction to the electrode
The most common side effects
- Intolerance to the feeling of CEFALY on the forehead: 1.25%
- Sensation of fatigue during and after the session: 0.65%
- Headache after one session: 0.52%
- Irritation of the skin on the forehead: 0.22%
The magnetic field right next to the Cefaly is below 0,2µT (i.e. 2mG). This value is negligible compared to the field generated by an electric shaver (typically between 15 and 1500µT when measured at 3cm) or by an hair dryer (typically between 6 and 2000µT).
Neurostimulation has been used for more than 20 years with devices that are implanted in the head and which work constantly for several years. The only problems encountered with implantable neurostimulators relate to the surgical procedure involved to implant them, and never to the neurostimulation technique itself. This is why this technique is regarded as exceptionally safe. Cefaly's great breakthrough is to allow this technique of neurostimulation to be used in an external and gentle manner.
PRE-EXISTING CONDITIONS
CEFALY is not contraindicated in the presence of a spinal cord stimulator. For safety reasons, we recommend the following conditions are met prior to use with CEFALY: (1) ensure all metallic or device portions of the spinal cord stimulator are below the level of the 2nd cervical vertebrae or C2 level and (2) if possible, turn off the spinal cord stimulator when the CEFALY device is in use.
CEFALY was not studied in patients with known brain lesions. The effectiveness and safety of CEFALY for patients with certain brain lesions (stroke, hemorrhage, tumors, etc.) is not known. Brain lesions are a very non-specific term and can vary widely in their cause, pathology, and prognosis. If you have brain lesions, we recommend that you discuss the risks and benefits of starting CEFALY with a neurologist.
If you have a history of any of these conditions, it is best to discuss them with your healthcare provider prior to starting CEFALY treatments.
The effectiveness and safety of CEFALY for Trigeminal Neuralgia is unknown. CEFALY has shown effectiveness and safety for:
- The rescue (acute) treatment of migraine attacks with or without aura in patients 18 years of age or older.
- The prevention of recurring (episodic) migraine in patients 18 years of age or older.
Allodynia is a symptom consisting of increased sensitivity to touch. People with allodynia may perceive painful sensations from objects or items that do not typically cause pain. Allodynia can affect your tolerance to nerve stimulation treatments.
If you experience allodynia, you should discuss these symptoms with your healthcare provider before starting CEFALY treatments. We recommend people with allodynia become familiar with the intensity stabilization function before using the CEFALY device.
The effectiveness and safety of CEFALY for vestibular migraine is unknown. CEFALY has shown effectiveness and safety for:
- The rescue (acute) treatment of migraine attacks with or without aura in patients 18 years of age or older.
- The preventative treatment of recurring (episodic) migraine in patients 18 years of age or older.
CEFALY is FDA-approved for the preventative and acute treatment of episodic migraines. If you have chronic migraine, it is best to discuss with your healthcare provider prior to starting CEFALY treatments.
CEFALY has not been systematically studied in the treatment of intractable migraines. If you suffer from intractable migraine, it is best to discuss with your healthcare provider prior to starting CEFALY treatments.
Migraines may involve a variety of headache locations and patterns. The majority of migraine headaches start or affect one side of the head. If you experience headaches that start in the back of your head, or if your headaches have an unusual location or pattern, it is best to check with your healthcare professional to exclude other causes of your headaches prior to starting CEFALY treatments.
The effectiveness and safety of CEFALY treatments for headaches in patients with Traumatic Brain Injury are unknown. If you have headaches associated with a history of Traumatic Brain Injury, it is best to discuss with your healthcare provider prior to starting CEFALY treatments.
Epilepsy or recurrent seizures is not a contraindication for CEFALY treatments, however, CEFALY treatments were not specifically studied in patients with epilepsy or a history of seizures. Additionally, CEFALY treatments are contraindicated in patients with metal components or devices inside or surrounding the skull. If you have a history of epilepsy surgery, including placement of a vagal nerve stimulator (VNS), you should discuss it with your epileptologist or neurosurgeon prior to starting CEFALY treatments.
People with a history of herpetic outbreaks involving the head or face may have difficulty tolerating the stimulation with CEFALY treatments. If you have a history of herpetic infections/Shingles, please discuss with your medical provider before starting CEFALY treatments.
The effectiveness and safety of CEFALY in patients with an intracranial tumor or cancer is unknown. If you have a history of these conditions, it is best to discuss with your healthcare provider prior to starting CEFALY treatments.
CEFALY is NOT contraindicated in pregnancy. However, the safety of CEFALY treatments in pregnancy has not been established in clinical studies. You should consult your healthcare provider prior to CEFALY treatments if you are planning to become pregnant or are pregnant.
The effectiveness and safety of CEFALY treatments in patients younger than 18 years of age is unknown. If your child experiences migraines, we recommend that you discuss with your child’s healthcare provider for any further questions regarding use in persons under the age of 18.
There are no contraindications of CEFALY treatments with other medications. Some CEFALY users may experience sleepiness with treatments. Therefore, caution is advised for patients starting CEFALY who are also taking sedative medications.
There are no contraindications of using CEFALY for migraine treatment in patients with fibromyalgia. If you have a history of fibromyalgia, please discuss with your healthcare provider prior to starting CEFALY treatments.
Most migraine headaches start before 50 years of age. New-onset headaches after age 50 raise the possibility of non-migraine-related causes. If you started experiencing migraines after age 50, we recommend an evaluation by a healthcare provider prior to starting CEFALY.
The effect of CEFALY treatments on migraine auras is unknown. People with migraine aura may report benefit with ACUTE CEFALY treatments as they can identify and treat impending migraine attacks early.
USAGE
Each CEFALY ACUTE treatment lasts for 60 minutes. CEFALY ACUTE treatments are best utilized early in the migraine attack or during migraine aura(s). The CEFALY ACUTE treatment can be used consecutively for up to two (2) sessions (i.e., 2 hours of ACUTE treatment). The effectiveness and safety of CEFALY ACUTE treatments, greater than 2 hours per day, has not been established in clinical studies.
Daily CEFALY PREVENT treatment (20-minute duration) can be used the same day as CEFALY ACUTE treatments.
The effectiveness and safety of using CEFALY treatments in any other capacity other than what is in the user manual have not been established in clinical studies.
The 20-minute CEFALY PREVENT treatment option is to be used once daily to reduce the frequency and intensity of migraine symptoms. This treatment option acts as a preventative. Like a medication you take daily, CEFALY PREVENT treatment sessions should be used daily to see long-term results.
CEFALY PREVENT 20-minute daily treatments can be used anytime during the day or evening. A common side effect of CEFALY treatments includes sleepiness after use. Many migraine sufferers may have trouble falling asleep and therefore prefer CEFALY PREVENT treatments prior to bedtime to transition to sleep. Though we encourage you to rest during therapy, CEFALY treatments should be avoided while sleeping.
Though clinical studies have demonstrated efficacy / results with CEFALY using the maximum stimulation intensity, you may experience migraine relief using CEFALY at lower stimulation intensities. When starting CEFALY treatments, the target intensity will vary from person to person. In general, the stimulation should be comfortable / tolerable without adverse effects for the entire duration of the treatment. The goal is to reach long and short-term migraine relief with a tolerable level of stimulation. Over time, your nerves may become accustomed to the stimulation intensity, and you may consider gradually allowing higher treatment intensities. It is common for headaches to become slightly worse during the initial minutes of CEFALY treatment; however, the treatments should not be painful before stabilization. Please refer to our instructional video “Controlling the Intensity.”
In clinical studies, once the full simulation is tolerated, relief from migraine attacks using the CEFALY ACUTE treatments are expected within the first five treatment sessions. CEFALY ACUTE often provides migraine relief as soon as the first treatment session. In a clinical trial, 29% of migraine sufferers reported complete migraine relief after 1-hour initial use of CEFALY ACUTE treatment. In the same study, 63% of migraine sufferers experienced a 50% or more reduction in their migraine severity pain at 1 hour after initial use of CEFALY ACUTE treatment. In addition, the effect of reduced average migraine severity was sustained over a 24-hour period.
For CEFALY PREVENT treatments, some users begin to see benefits within 6-8 weeks; however, most users see results by three months.
If you use CEFALY regularly, you may eventually perceive the stimulation from the device to be less intense over time. This is normal and your device is not defective or malfunctioning. For experienced CEFALY users who are accustomed to the lower stimulation intensities, the intensity can be rapidly increased by pressing and holding the device button after the first 10 seconds of starting a treatment. Please refer to the user manual section titled “Manually Increasing the Intensity During First 14 Minutes.”
It is always good practice to ensure proper application of the adhesive pads and CEFALY device. Please refer to the video “How to Use the CEFALY migraine treatment device”.
For people with migraines, it is common to have increased sensitivity on one side of the head. If the device is properly applied, the device is still working even though you may not feel the treatment equally on both sides.
In all clinical trials, there were no serious adverse side effects, and all side effects of CEFALY ACUTE and PREVENT treatments were temporary and completely reversible. The long-term effects (greater than 90 days) of regular CEFALY use are unknown.
The longest clinical trial of CEFALY PREVENT treatments evaluated safety over 90 days or 3 months. There were no adverse events or side effects reported during this study. In a survey of 2,313 CEFALY users across three countries, side effects were reported in 4.3% of users during a 40-day at-home trial period. All reported side effects were minor and fully reversible.
If the electrodes are stored, applied, and changed according to the recommended usage, skin dryness at the electrode site may be related to frequent electrical stimulation.
If you experience local skin dryness with repeated stimulations, refrain from additional CEFALY treatments until the rash or dryness completely resolves. Skin moisturizers may help facilitate skin recovery and should be continued once CEFALY treatments are resumed. If you experience repeated skin dryness, hypoallergenic electrode pads are available via special order or through your CEFALY representative. Allergic skin reactions to the electrode pad are rare and may also improve with hypoallergenic electrode pads. You can contact a CEFALY representative via info@CEFALY.us.
Over time, electrodes lose water and dry out. Dry electrodes are unable to properly support the stimulation produced by the CEFALY device. Also, the loss of adhesion may indicate there is oil/ dead skin cells/ dirt on the electrode gel surface. Electrodes that do not completely adhere to the skin may result in improper stimulation and potentially lead to injury. To avoid injury and ensure adequate performance of the electrode and device, ensure that the electrodes are moist, without cracks or gaps prior to usage. Proper forehead cleaning and preparation before CEFALY use may improve longevity of electrode use.
Paresthesia (pins-and-needles sensation) of the head and stimulation area is the most common symptom encountered when starting CEFALY treatments.
If you are first starting CEFALY, it is important that you understand the stabilization feature of the CEFALY device prior to use. Please refer to our instructional video “Understanding the intensity.” Early stabilization is your primary tool to minimize uncomfortable paresthesia sensations when starting CEFALY treatments. Over time, the tolerance to stimulation may improve, allowing for higher intensities without uncomfortable paresthesia sensations.
If you experience paresthesia during CEFALY use, the sensations should subside within a few hours after CEFALY use. If symptoms of paresthesia persist for greater than 24 hours, stop CEFALY treatments and contact us at info@CEFALY.us.
Yes. Implanted cardiac devices (i.e., pacemakers, loop monitors, defibrillators) are listed as contraindications to CEFALY use according to the US Food and Drug Administration.
CEFALY has not been studied in patients with implantable cardiac devices, therefore the contraindication is precautionary. The risk of inference of the CEFALY device with implanted cardiac devices is unknown. Though the risk is unknown, the potential for profound consequences of electrical dysfunction of an implantable cardiac device is a primary factor leading to the contraindication labeling.
If there are specific concerns regarding this contraindication, we recommend you discuss with your cardiologist and / or neurologist to determine the most appropriate and individualized treatment option and alternatives.
In general, there are two types of ventriculoperitoneal (VP) shunts; programmable and non-programmable. Programmable VP shunts contain metallic or electrical components. The use of CEFALY is contraindicated with programmable VP shunts. Non-programmable VP shunts are commonly made from silicone and are safe with the use of CEFALY. It is always a good practice to check with your healthcare provider prior to using CEFALY to ensure your VP shunt does not contain any medical or electrical components.
In general, there are two types of ventriculoperitoneal (VP) shunts; programmable and non-programmable. Programmable VP shunts contain metallic or electrical components. The use of CEFALY is contraindicated with programmable VP shunts. Non-programmable VP shunts are commonly made from silicone and are safe with the use of CEFALY. It is always a good practice to check with your healthcare provider prior to using CEFALY to ensure your VP shunt does not contain any medical or electrical components.
It is recommended for patients to remove any metallic jewelry around their head or neck region whenever using the CEFALY device. This includes tongue piercings, eyebrow piercings, and nose rings.
The metal component in the metal plates in the electrodes are iron plates coated with zinc.
There are trace amounts of lead in the electroplating surface of the metal connectors on the USB cable that are within the minimum allowable standards. There is no lead found on any other external part of the device or cable.
CEFALY defines the head as anything above and including the upper jaw.
If you have implanted metal objects in your lower jaw, you may use CEFALY, however we strongly recommend that you discuss and follow your healthcare provider in the event you experience any side effects.
If you have implanted metal objects in your upper jaw, CEFALY is contraindicated. Dental fillings are not consistent with metal implants.
Dental implants can vary in their composition and placement. CEFALY is not recommended if you have implanted metallic components in the head. If you have implanted metal objects in your lower jaw, you may use CEFALY, however we strongly recommend that you discuss and follow your healthcare provider in the event you experience any side effects. If you have implanted metal objects in your upper jaw, CEFALY is contraindicated. Dental fillings are not consistent with metal implants.
For more specific information regarding the presence of metal with dental implants, please contact or discuss with your dentist.
The presence of metal dental fillings, or dental amalgam, should not interfere with CEFALY treatments for migraines. If you experience dental pain with CEFALY treatments, we recommend stopping CEFALY sessions and consulting with a dentist or neurologist for further evaluation.
DIFFERENT TYPES OF HEADACHE
The effectiveness and safety of CEFALY for cluster headaches is unknown. CEFALY has shown effectiveness and safety for:
- The rescue (acute) treatment of migraine attacks with or without aura in patients 18 years of age or older.
- The prevention treatment of recurring (episodic) migraine in patients 18 years of age or older.
CEFALY has a primary indication for migraine treatment, however, CEFALY may also be effective in treating tension-type headaches. Please discuss with your healthcare provider for additional information.
The effectiveness and safety of CEFALY for daily persistent headache has not been established in clinical studies. If you experience daily persistent headache, please discuss with your primary care provider prior to starting CEFALY treatments.
EYES, EARS & HEARING AIDS
If you experience blurry vision in one or both eyes during CEFALY treatments, stop the treatment session and contact your healthcare provider. Blurry vision is rarely reported with CEFALY use; however, should you experience these symptoms, do not continue to use the treatment until you contact a medical provider regarding your symptoms. All users experiencing blurry vision are also encouraged to contact our medical department for additional information and guidance. Once the blurry vision has subsided, we would strongly recommend you try using the CEFALY device at a lower intensity setting. You should press the button a second time to stabilize the intensity before it becomes uncomfortable.
Some descriptionEyelid heaviness, eye twitching and eyelid paresthesias (pins and needles sensation) are common during CEFALY treatments. During initial treatments, you may notice difficulty keeping eyelids open. Please refrain from attempting to overcome or force your eyes open during initial treatments. We recommend seeking a quiet and relaxing location when first starting CEFALY treatments.
Please keep in mind that the stimulation intensity SHOULD NOT be painful. Prior to starting CEFALY treatments, it is critical to understand the stabilization feature to limit the intensity of stimulation. Please refer to our instructional video “Controlling the intensity.” Early stabilization may assist in facilitating tolerability of the stimulation intensities especially when first starting CEFALY treatments.
Tinnitus (ringing in the ears) is a potential side effect with CEFALY use occurring in approximately 1% of patients in clinical studies. Use of the stabilization feature may help to mitigate this symptom if encountered during treatment. Please refer to our instructional video “Understanding the intensity”.
Symptoms of tinnitus after CEFALY treatment should subside or improve within 24 hours. If symptoms of tinnitus persist or do not improve after 24 hours, please refrain from CEFALY treatments until symptoms completely resolve.
If you have a cochlear implant, we recommend discussing it with your ENT provider prior to starting CEFALY treatments. Implanted metallic or electronic components of the head are a contraindication for CEFALY use.
The FDA has listed any implantable metal or device as a contraindication to using CEFALY. This includes the implanted portions of hearing aids, even if the metals are of those considered poor conductors of electricity (e.g., aluminum). Therefore, we cannot recommend the device in this scenario.
OTHER TREATMENTS
There is no contraindication for using CEFALY with Botox injections. If you are interested in CEFALY treatments and Botox injections, we recommend that you discuss both therapies with your healthcare provider.
If you already receive Botox injections for chronic migraine, we recommend waiting 48-72 hours after an injection before placing a CEFALY electrode and starting CEFALY treatments.
If you are new to both CEFALY and Botox treatments, we recommend starting one therapy at a time and waiting at least three months before beginning the other treatment.
Currently, there are no clinical trials (medical studies) directly comparing the effectiveness and safety of CEFALY treatments to other acute or preventative migraine treatments. There are no contraindications of using CEFALY with other neuromodulation devices. Please discuss with your healthcare provider before using multiple headache treatment devices.
Yes. There is no contraindication of using CEFALY and CES on the same day. If you are using both therapies, please consider the following:
- Avoid using both CEFALY and CES at the same time.
- Both CEFALY and CES may contribute to drowsiness or sleepiness. Starting one therapy at a time may be best to determine how you respond to each treatment.
CEFALY use with transcranial magnetic stimulation (TMS) has not been studied in clinical trials. If you anticipate a finite number of TMS sessions, it is recommended to complete TMS therapy prior to starting CEFALY therapy.
If you have prior exposure to TMS and have no side effects related to the treatments, then starting CEFALY may certainly be considered. If you anticipate ongoing TMS therapy, we recommend discussing it with your prescribing healthcare provider prior to starting CEFALY treatments with TMS.
Relating to Common Adverse Events
If you are experiencing eye, ear, jaw, and/or tooth pain, we suggest you stop using the device until symptoms subside.
Once the pain has subsided, we would strongly recommend you try using the CEFALY device at a lower intensity setting. After selecting and starting your treatment, you should press the button a second time to stabilize the intensity before it becomes uncomfortable.
If symptoms persist, stop CEFALY treatment and discuss these symptoms with your doctor or neurologist for further evaluation and treatment.
If you are experiencing local pain, a pins and needles sensation, or numbness, try using the CEFALY device at a lower intensity setting. After selecting and starting your treatment, you should press the button a second time to stabilize the intensity before it becomes uncomfortable.
Any feeling of shocking, zapping, or electrical sensations that result in rashes, marks, or dents is an indicator that a new electrode should be used. Ensure you are washing your face with soap and water before placing the electrode on your forehead. When placing the electrode on your forehead, refer to the user manual for proper electrode placement. Run your fingers across the surface of the electrode, ensuring there is strong adhesion between the electrode gel and your forehead skin.
*See "Shocking, Zapping, Electrical Sensations that result in rashes, marks, dents on the Forehead'*
*If applicable, also see FAQ on Allodynia*
If your headache or migraine has worsened or changed, we strongly recommend you try using the CEFALY device at a lower intensity setting. After selecting and starting your treatment, you should press the button a second time to stabilize the intensity before it becomes uncomfortable.
If symptoms persist, or there are further questions, we recommend you schedule an appointment with your doctor or neurologist for further evaluation and treatment.
One of the known side effects of using CEFALY is a tendency toward sleepiness. Therefore, CEFALY cannot be used while driving, operating machinery, or during activities that can put you at risk of injury. If you are experiencing excessive and persisting sedation or changes in mental status, please refrain from additional CEFALY treatments until you are evaluated by a medical provider. In the meantime, we can pass your contact information along to our medical affairs team so you can discuss your symptoms with them.
*See Blurry Vision Q&A*
Oftentimes, when customers experience nausea and vomiting or twitching of the face, mouth, eyes, and/or cheeks, the causes are secondary to the migraine attack itself. However, if the issue persists while using CEFALY, we recommend you schedule an appointment with your doctor or neurologist for further evaluation and treatment.
*See FAQ on Tinnitus*
We do not have data regarding the symptoms of changes in heart rate and difficulty breathing while using CEFALY. We suggest you schedule an appointment with your doctor or neurologist for further evaluation and treatment.
Reports of changes in smell and taste are transient and fully reversible adverse effects with CEFALY treatments.
We recommend stopping treatment until your smell and taste return. Once that happens, we strongly recommend you try using the CEFALY device at a lower intensity setting. After selecting and starting your treatment, you should press the button a second time to stabilize the intensity before it becomes uncomfortable.
If symptoms persist, or there are further questions, please stop using the CEFALY device and schedule an appointment with your doctor or neurologist for further evaluation and treatment.
We don't have data regarding CEFALY and people with a history of hemorrhagic stroke or aphasia. We would strongly suggest you contact your neurologist prior to starting treatments with CEFALY.
If your neurologist says it is okay to use CEFALY, it is important that you familiarize yourself with how to stabilize the intensity of your treatment. Please keep in mind, CEFALY should never be painful. You should stop the intensity by pressing the button a second time before it becomes uncomfortable. It is more important that you keep it on for the full duration of the treatment (60 minutes for ACUTE or 20 minutes for PREVENT), than the level of intensity you use.
- Allergic dermatitis is an allergic reaction of the skin, resulting in swelling, bumps, burning, itching, and vesicular formations due to a substance the skin is reacting to.
- Chronic migraines are recurring migraines where the frequency of migraine headaches occurs 15 or more days per month.
- Episodic migraines are recurring migraines where the frequency of migraine headaches occurs less than 15 days per month.
- Migraine Aura is a series of sensory disturbances that happen shortly before a migraine attack. These disturbances range from seeing sparks, bright dots, and zig zags to tingling on one side of the body or an inability to speak clearly, and usually last 20-60 minutes. (American Migraine Association)
- Preventative migraine treatments are migraine therapies used on a scheduled basis to suppress or decrease the number and / or severity of migraine attacks.
- Rescue (acute) migraine treatments are migraine therapies used after a migraine attack or aura has started.
Chou, D. E., Yugrakh, M. S., Winegarner, D., Rowe, V., Kuruvilla, D., & Schoenen, J. (2018). Acute migraine therapy with external trigeminal neurostimulation (ACME): A randomized controlled trial. Cephalalgia, 1- 12.
Schoenen, J., Bandermissen, B., Jeangette, S., Herroelen, L., Vandenheede, M., Gerard, P., & Magis, D. (2013). Migraine prevention with a supraorbital transcutaneous stimulator: A randomized controlled trial. Neurology, 8(8), 697-704.
Magis D, Sava S, d'Elia TS, Baschi R, Schoenen J. Safety and patients' satisfaction of transcutaneous supraorbital neurostimulation (tSNS) with the CEFALY® device in headache treatment: a survey of 2,313 headache sufferers in the general population. J Headache Pain. 2013 Dec 1;14(1):95.

















